Beautiful Work Tips About How To Draw Covalent Bonds

Contents:0:08 introduction 0:39 h21:25 hcl2:23 cl2.
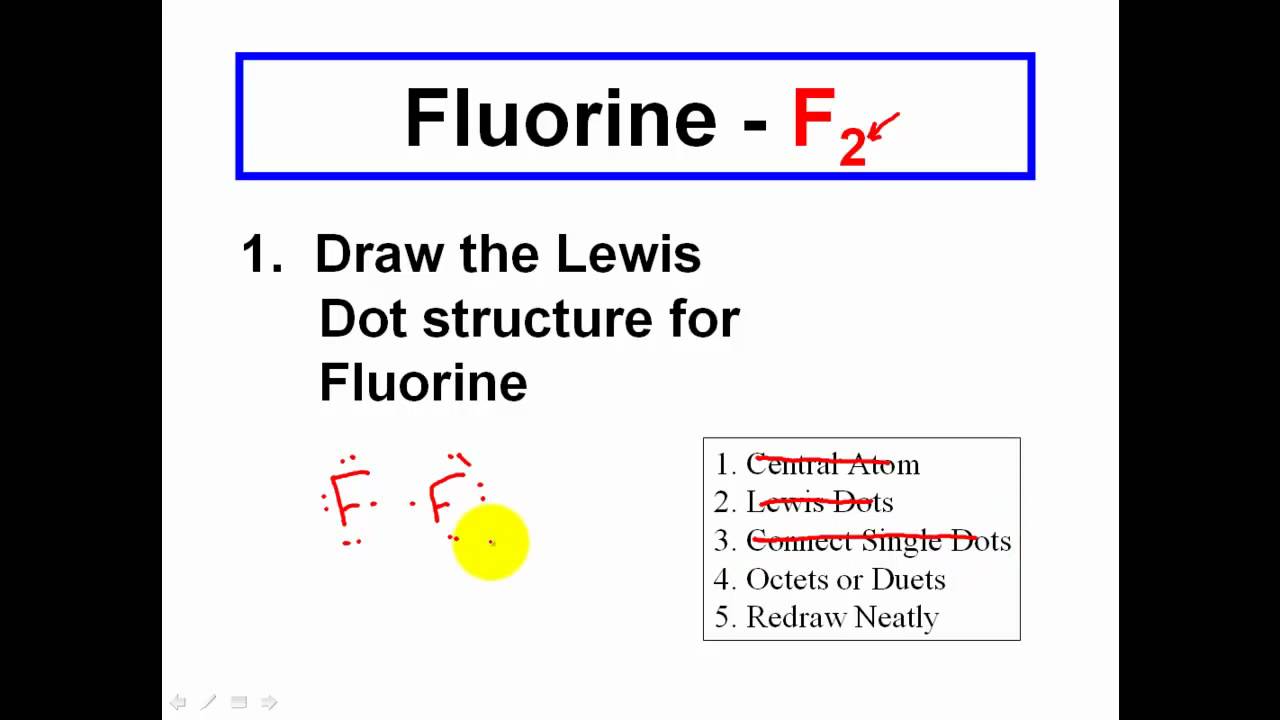
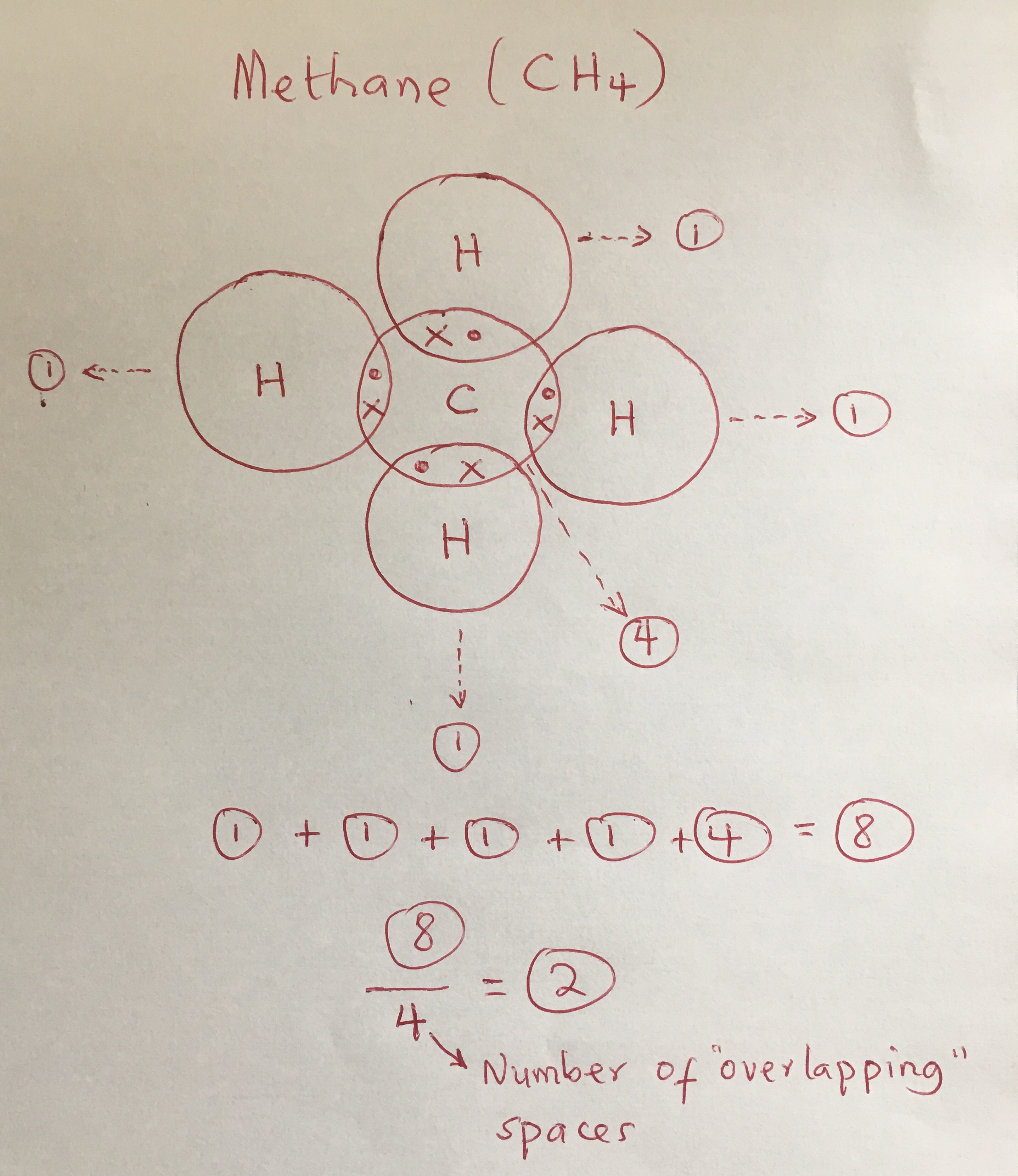
How to draw covalent bonds. Formation of covalent bonds we draw dot and cross diagrams with overlapping valence electron shells, to illustrate how covalent bonds are formed. The diagrams below illustrate the. Determine the number of valence electrons available.
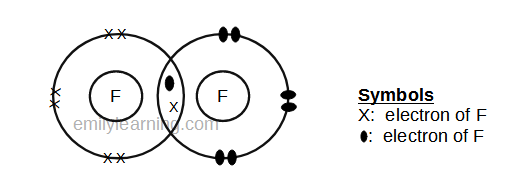
There are three shared spaces between the circles, so add a dot and. In these diagrams, we use circles to represent the electron shells. Draw a lewis dot structure for the valence.
Water is a polar covalent molecule. It is often easiest to draw circles at 90° or 180° to each other. It is often easiest to draw circles at 90° or 180° to each other.
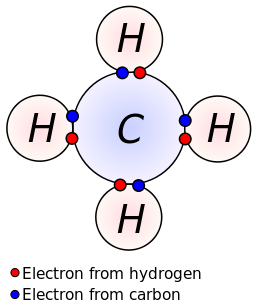
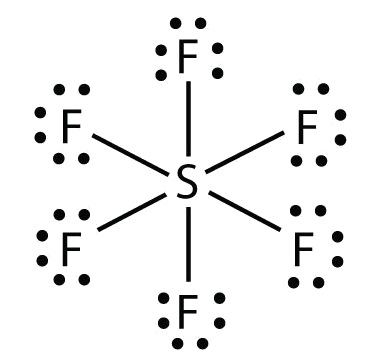
In covalent bonds, atoms share electrons. Covalent bonding can be achieved in two ways: Determine the number of electrons necessary to satisfy the octet (or duet) rule with no electron sharing.
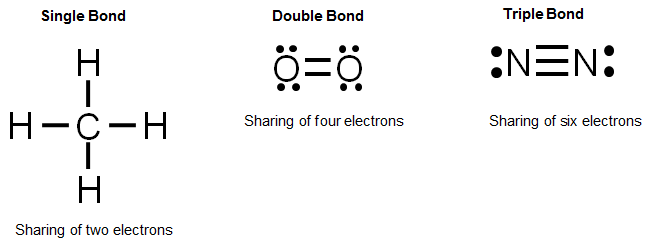
Nitrogen is in group 5 so it forms three covalent bonds. A single covalent bond is when two atoms share a single pair of electrons. There are three shared spaces between the circles, so add a dot and.
There are different kinds of covalent bonds: How to draw covalent bonds. Sharing of electrons between atoms of the same kind e.g.