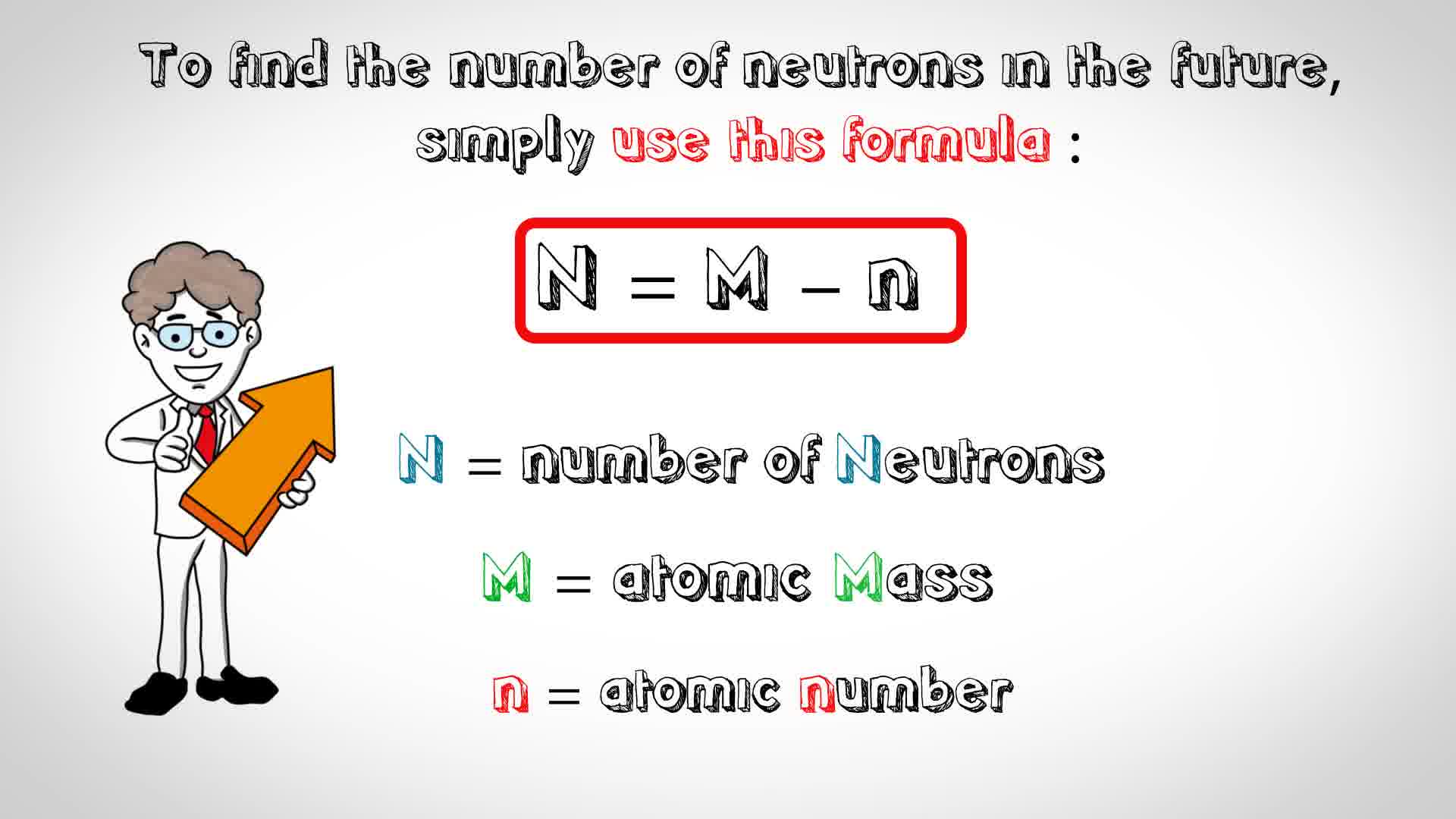Simple Tips About How To Find Out The Number Of Neutrons
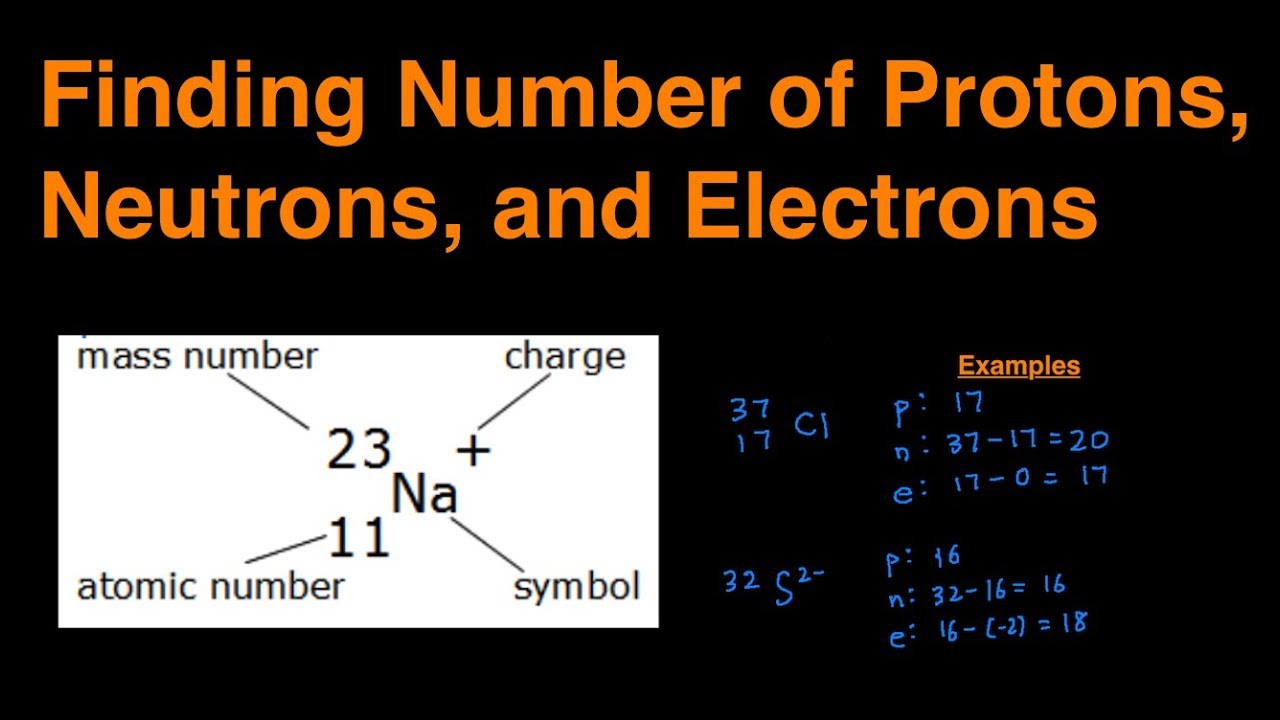
This chemistry video tutorial explains how to calculate the number of protons, neutrons, and electrons in an atom or in an ion.
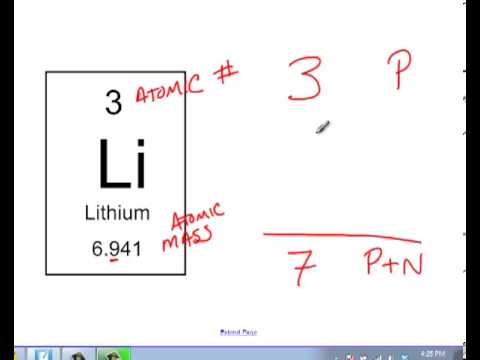
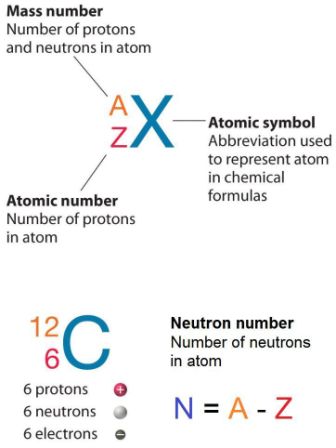
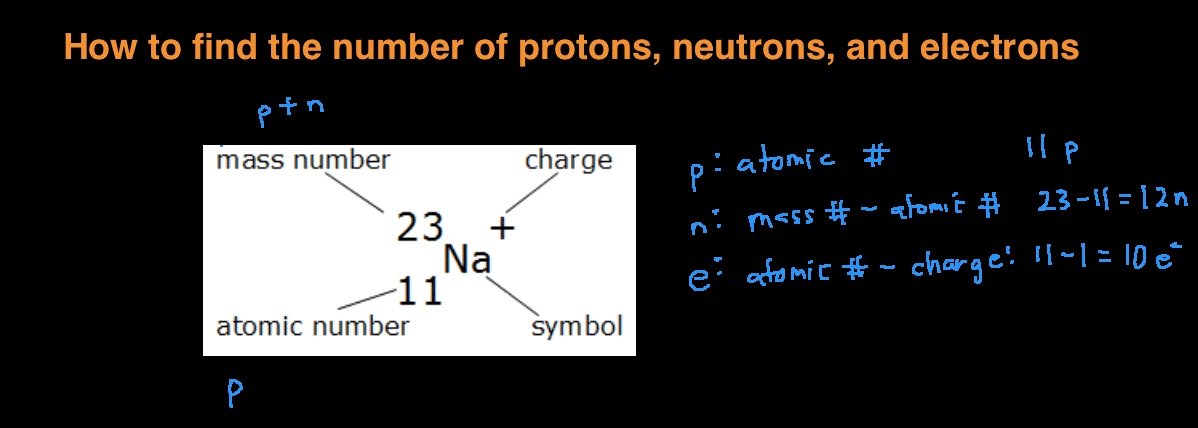
How to find out the number of neutrons. When we write the symbol for an atom, we can place its mass number at the top left and its atomic number at the bottom left. It also explains the differe. How to calculate the number of neutrons in an atom given the information provided by the periodic table of elements
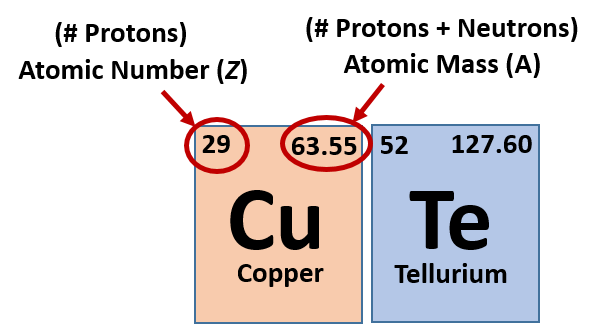
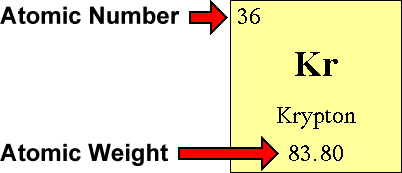
The relative atomic mass is the number of. Finding the number of neutrons in a regular atom locate the element on the periodic table. Find the element’s atomic weight.
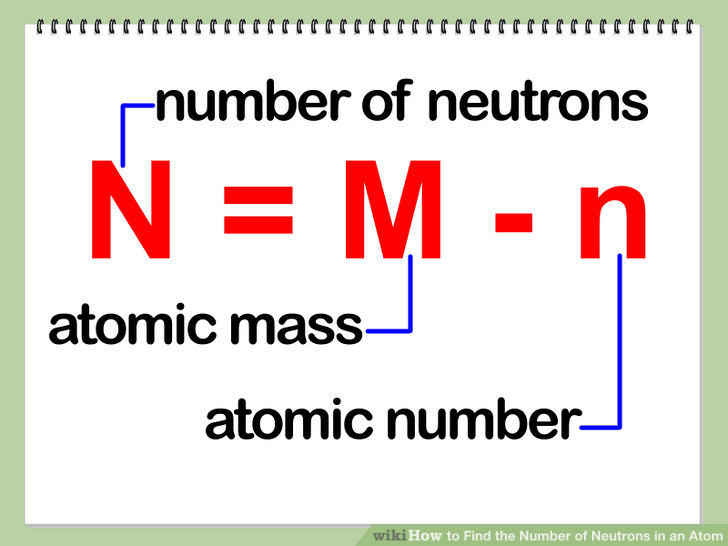
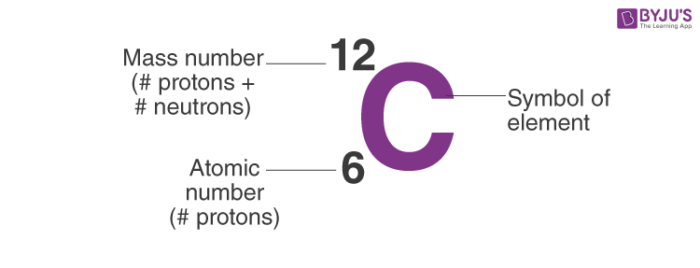
Rules to finding number of protons, neutrons, and electrons. The number of neutrons in an atom can be figured by subtracting the atomic number from the mass number. Identify the mass number of the isotope.
How to find electrons, when an atom is ionized, it loses one or. 6) subtract the atomic quantity from the atomic mass. Find the element’s atomic number.
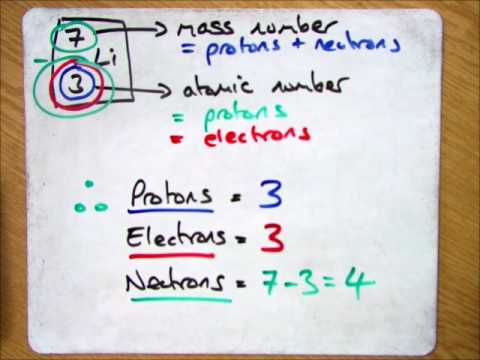
You can use these numbers to calculate the number of. How do you find the neutron number? To find the number of neutrons, subtract the number of protons from the mass number.
Almost all atoms contain three main particles: To calculate the number of neutrons in the nucleus of an atom is simple. The mass number can be found in the isotope's name.